Leaderboard
Popular Content
Showing content with the highest reputation on 07/15/2022 in all areas
-
Hi guys, My business partner Mark Jones and I have recently taken in a large collection of swords and I have been listing some to my website. Yesterday I put up a fine Jumonji Yari (in polish) and I just finished listing a long katana (75.6 cm) with Tokubetsu Hozon paper, signed Echizen no Kami Minamoto Sukehiro (Tsuda Sukehiro). https://www.japaneseswordbooksandtsuba.com/store/swords/q682-long-katana-tsuda-sukehiro-tokubetsu-hozon And there is more to come: some very nice pieces (a large shinsakuto gifted to a Sumo star and a fine daito from the Bizen Kozori School with wonderful itomaki no dachi koshirae come to mind). All are in polish and most have papers. There are good swords for entry level (beginners) also: quality work that won't break the bank. If you have a minute have a look and please keep checking back; I'll be listing more in the next few days. Stay well, Grey6 points
-
Excellent! When I started out and told my teachers that I was interested in Shinshin-to they all laughed, "You have to study Koto!" so you're in good company. It is a huge area indeed but that is what makes it all so enjoyable. Roughly there are four paths; Art Appreciation - study of the swords as art and kantei is the tried and true method for gaining knowledge in this area. Craftwork - construction, molding, forging, polishing, all the things that attract the "Hammer Monkeys". Fittings - mountings and all the bits and bobs that adorn the sword. History - the context, politics, fads, art and societies that influenced the sword. (I fall mostly into this last category) However you will see overlap in all these areas and long time students will understand you have to have knowledge, at least a little bit, in each of these areas for true appreciation. Ms. Halchaks book contains much the same information as what you already have but presented in interesting ways that may help cement some in your minds eye that others do not. Lonnie Kapps book is a must for everyone - no other book presents the processes of construction, forging and polishing in an understandable format like The Craft of the Japanese Sword. Of course if you want to delve deeper into this area his newer books provide lots of great information. On kantei in a swordless world - for more than ten years I studied the paper kantei (Shijo kantei) and monthly kantei notes in the NBTHK and NTHK magazines - when I couldn't read Japanese and I had no ready access to live blades. Once I got to Japan I had to learn a whole new set of kantei rules when looking at the real thing but that early study helped immensely. I recommend you join the NTHK and NBTHK and any other organization offering regular "paper kantei" these challenges help you to see swords the way the Japanese do and cement the terms and their importance in your minds eye. Do this and when the Society does start meeting again you'll be ready! -tch5 points
-
Ford is indeed the man for the job, currently he is engaged upon the conservation of a nunome zogan box for me. His knowledge of Japanese metals and their care is unparalleled.4 points
-
4 points
-
Yes, C96 to be exact and commonly called the "broomhandle" by collectors. The Japanese acquired so many from the Chinese that they adopted it as the 「モ」式大型拳銃 in 1939! Good to see Mal branching out into the heavy artillery! Mauser C963 points
-
While I have been spurred to action. I've previously mentioned I'm rather dubious about calling a number of the less common colours a 'camouflage'. Too often casual statements without any basis at all in documentation become 'fact' when read online. Especially when there is no disclaimer that it is an opinion or guess, or the guess is stated as a fact. While I was looking for examples of plexiglass modifications in LaBar's excellent book on Japanese bayonets (for a different thread and topic), I found the souvenirs thread. Wouldn't you know, some of the examples have the same colours of paint and unusual mixes (like black with gold highlights) we have seen on 95s. Worth popping in here as an example at least of why we might find some fancifully painted 95s with period appropriate patina. On a separate note. Looking through the whole book, there are two observations I'd like to add. There seem to be a fair number of black painted bayonet scabbards. Now, I've been and remain dubious about a good majority of black 95 saya. The pertinent questions are: Factory or field painted? Originally painted black or refurbished later? Why is there is such a variation in quality of finish (sometimes black is over metal and no traces of other paint, but regularly there are traces of a previous colour or sometimes at worst, the original paint seems to have black slapped straight over the top) There are a number of modern repaints to black to muddle this further There is no official documentation at all about the use of black or any particular reason to use it ('supply' doesn't cut it. As Nick stated, the army had a ready supply of 'red bean' paint which was used on equipment) At this point I'd like to state that I tentatively support the idea that A LIMITED NUMBER of black saya may be period and a further limited number of these appear to possibly original, with no traces of a previous paint under the black. However, just like EVERYONE else with an opinion on these, there is no primary evidence nor reasoning explaining why black may have been used. That is something where we are still looking for answers. All we have are a mixed bag of examples, not very conducive to a neat explanation.3 points
-
3 points
-
To return to this point of agility Vs inertia and how easily people lose their lives when sharp/ pointy weapons are involved; there's a video circulating social media of an altercation (in a food court in Australia) where one man (holding a knife) swings at another man and makes contact with his neck... The man noticed the blood streaming from his neck, collapsed on the floor, and died very shortly thereafter. It's a horrible thing to see. I won't share the video here, for obvious reasons. It really is important that (if possible) people get a little bit of experience in boxing, kickboxing, etc and learn a proper guard. If you do have to face someone armed with a sharp weapon, you'll probably get stabbed and cut either way, but a solid guard and some training will at least ensure you properly protect the neck and have some chance of survival. This felt worthwhile to mention, but please delete if it's too far off topic.3 points
-
2 points
-
Good afternoon Alban, In the 19th Century, small boxes like this were sometimes used for small items of interest, often contained in a larger Cabinet of Curiosities (Wunderkammer). Later generations would repurpose them according to their requirement and Social standing. When I discovered this one, last year, it had been used for Turkish Cigarettes since the 1960's.2 points
-
Chris, Gold and Silver are not meaningfully different to other atomic nuclei from the perspective of particle physics. Nickel and all subsequent (i.e. higher atomic number) elements were formed via Supernova Nucleosynthesis (or perhaps in the core of Neutron Stars). The atomic nucleus of a stable element will not (ever) decay. Gold and Silver are no different from any other stable element in this regard. There are however unstable isotopes; Gold-198 for instance is a radioactive isotope of Gold which undergoes beta decay to stable Mercury-198 with a half-life of 2.697 days. Additionally, Gold can be Oxidised and fully dissolved in Aqua Regia; Gold can be Oxidised. No atomic nucleus is altered in any chemical reaction; the nuclei are only alerted in radioactive decay, fission and fusion. Chemical reactions only affect chemical bonds and the energy released or absorbed in the reaction is dependent on the difference in enthalpy of the bonds broken and the bonds formed. "When the universe is long gone", whatever that means, it would surely presuppose that the matter and energy would be long gone (including Gold and Silver). From a chemical perspective, oxidation of iron is fully reversible. This is what smelting is all about; extracting a metal from its ore via reduction, but this doesn't help much with regards to an object as the corrosion is permanent and the iron is now elsewhere. Oxidation is, technically speaking, loss of electrons. An atom (or atom group) loses electrons when it is oxidised - conversely, an atom or atom group that gains such electrons is reduced. In inorganic nomenclature, the oxidation state is represented by a Roman numeral placed after the element name inside the parenthesis or as a superscript after the element symbol, e.g. Iron(III) oxide. Combination of both half-reactions is what is termed as a Redox reaction, an electron transfer from one chemical species to another. Gold “does not oxidise” according to our experience because it ranks very high in the so-called electrochemical series (Standard electrode potential), a list of how easy it is to oxidise chemical species. As Gold is above oxygen (the commonest oxidiser), no oxidation occurs to it. However, the reaction with Aqua Regia does involve Oxidation of Gold to form Chloroauric acid (HAuCl₄) in solution via the following reaction: Au + HNO₃ + 4HCl ⟶ [AuCl₄]¯ + [H₃O]⁺ + NO + H₂O2 points
-
I imported a bunch of captured and warehoused WW2 weapons from China many years ago. There were both German and Chinese C96's of various variations. Yes, China had German ones, including full auto Schnellfeuers, Astra 702 and 703's etc etc. But the Chinese variations were many, and some bizarre ones. I can't say if that one is German or Chinese. Late period small ring..maybe German.2 points
-
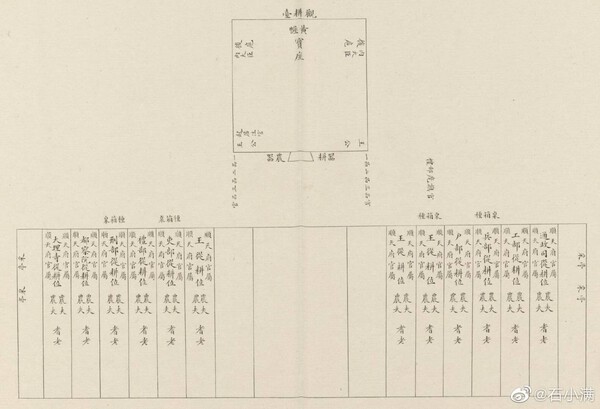
Looks like 御研 = sword polishing. Not sure of the pronunciation. "miken" I think. 耕觀 = probably the name of the polisher. Again, unsure of the pronunciation. "Kōkan" maybe. Nothing comes up in a search for that name.2 points
-
2 points
-
2 points
-
Brethren, Just stumbled across this site I haven't seen before: https://www.spoon-tamago.com/2020/02/05/eliza-scidmore-photographed-everyday-life-in-Japan-over-100-years-ago/?mc_cid=77cafa5ffb&mc_eid=962267c38c There are numerous 'by-way' tags of interest there too. Bestests, BaZZa.2 points
-
All are good, some are just quite late, but some are really decent in every aspect.1 point
-
1 point
-
I asked Nick Komiya over at WRF to take a look at this and above is his reply.1 point
-
I was trying to search 耕觀 first, but nothing came up, so I decided to try the reverse and see what it would be.かんぱい!1 point
-
1 point
-
David S., after March 1945, anything is possible in regards to Japanese swords. Starting in the last year of the war, all kinds of patriotic sword production took place. Type 98 sword with unusual mark on tang Wartime Japanese Radio Broadcasts Related To Swords1 point
-
The wrong order of characters my friend. In this case, 觀耕台 means "viewing platform" while I think the characters would more likely be in the order of 耕觀? Me thinks Trystan has been imbibing on 烧酒 shāojiǔ!1 point
-
Paz - not enough just to "buy" the books, you gotta read them! Memorize, cross-check, contrast and compare. This is an excellent start. Where you go next largely depends on your tastes, desires, unanswered questions that develop from your reading these introductory books. If you already have a particular area of interest we are happy to point you to references that will further your study. One precaution that I heard early on; "If you can't read it, you don't need it!" You can easily go bankrupt and get buried under all the great Japanese sword books out there but if you can't understand what they are telling you they will be of little help. Take your time, study. One way to solve this problem of course is to learn to read! -tch1 point
-
Aqua Regia does not only change the state of matter, the Gold is oxidised and has undergone a chemical reaction. The Gold can easily be reduced to return to pure Gold, but this is more than a change of state. A state change refers classically to solid, liquid, gas, and plasma. This is not a state change (i.e. melting the Gold to turn solid Gold into a liquid), but is a chemical reaction involving Oxidation of Gold; just as rust is formed through the Oxidation of Iron. In a chemical reaction none of the matter is used up (that would be a nuclear reaction). If we consider complete combustion of Methane: Methane + Oxygen → Carbon Dioxide + Water But this does not tell the whole story and could give rise to misunderstandings regarding conservation of mass (i.e. an assumption that mass is not conserved when in fact it is). The Stoichiometric equation which is properly balanced accounts for conservation of mass by including the ratios of the respective reactants and products: CH4 + 2O2 → CO2 + 2H2O Since: 1 mole methane = 12 +4 =16g 2 mole oxygen = 2×32 = 64g 1 mole CO2 = 12 + 32 =44 g 2 mole water=2 × 18 =36g From the balanced reaction: 1 mole(or 1 molecule or 16 g) of CH4 reacts with 2 moles (or 2 molecules or 64 g) of oxygen to give 1 mole (or 1 molecule or 44 g) of CO2 and 2 moles (or 2 molecules or 36 g) of water. For the gaseous system at STP (Standard temperature and pressure): 22.4l methane reacts with 44.8l of oxygen to give 22.4l of CO2 and 44.8l of water. No mass is gained or lost. No nucleus can be "destroyed" other than by radioactive decay, fission, fusion or potentially annihilation; this is true of every element and is in no way specific to Gold and Silver. Conservation of mass is a fundamental premise of all Chemistry and is in no way unique to Gold and Silver; I don't understand where this misconception is coming from.1 point
-
Thank you guys for the answers ☺️. Yes Thomas, that makes a lot of sense. I just wanted to understand whether or not a piece of koshirae could be, even very remotely, linked to a particular family thanks to the mon represented on it. Thanks again for your inputs, much appreciated!1 point
-
Gentlemen, I am suspecting I am a bit out of my comfortable depth, but: 1. Silver is not widely used in electronics industry despite having many advantages in terms of conductivity related metrics over gold: because it oxidizes. With jewelry it is not an issue at all as the layer is very thin and removed by any rubber eraser, but in electronics that would be difficult. In jewelry they indeed use "oxidized silver" which is black silver sulfide rather than silver oxide. This is also a common terror of houses build next to coal power plants or similar industry - copper and silver there has a sulfide problem. Its rather common in the UK apparently, but its not a universal problem. 2. Gold does not oxidize but its never used in jewelry as pure metal. Very old gold will loose a bit more gold due to its high plasticity and will become more copper-lead. So yes, with some skill and good color perception you can distinguish old Kofun period's or say Scythian gold because it has content which people typically don't use and their copper base was rather dirty. Gold is one of the most plastic metals and can actually wear and tear a lot.1 point
-
Definitely. Do NOT try anything yourself. Not sure if he's taking on work anymore, but drop @Ford Hallam a message for advice.1 point
-
The Chinese did buy Mauser C96's from Germany at various stages, along with indigenous production (of varying quality).1 point
-
1 point
-
As Kiipu said, apparently captured a lot from the Chinese when they invaded Manchuria (I believe in 1931?). They liked them so much the ended up also acquiring some more later on, mostly for civil defense forces or so I've read. Do just want to make clear this is a Mauser C96 copy made in China and has nothing to do with Germany.1 point
-
I've been bothered by this muddying of the waters regarding corrosion and rusting. The actual physical and chemical process whereby iron and steels is converted into any of the corrosion products I listed as 'rusts' in my previous post is called, by real metallurgical scientists, the corrosion process. We can have our own, tsuba/tosogu specific understandings of patina etc. and personally I need to make that distinction sometimes because that's a big part of my own particular work. But : The action on the metal is 'the corrosion process' and the result of this process is 'the corrosion product' or rust. Sometimes this rust can be made into a stable and attractive finish we enjoy as patina.1 point
-
Dan, your first source is not always correct (I won't read it all). I would be critical with the content. ...It is a slow process (what is exactly 'slow'?) Rusting is a type of corrosion Corrosion includes rusting. (Correct, please remember that) Rust is of red, orange color and the end product is flaky in nature (no, that is no complete answer. The appearence of 'rust' may depend on the iron type, and the colour range extends up to black. Iron has three oxides with different colours, and these combine to 'rust'. I would like to add that corrosion does not occur on ceramics, and corrosion on iron alloys is basically a chemical reaction with oxygen. Water (= moisture) is necessary in the formation of iron oxides ( x Fe{II}O . y Fe{III}2O3 . z H2O ). Try to understand what Ford wrote; there is not much to add.1 point
-
I didn't suggest they were interchangeable at all. Nice summation of exactly what I wrote; "Corrosion in fact covers the damage caused by rust, and that's really what we're looking at here. It is perfectly correct to speak technically about a corroded steel structure and to distinguish it from a merely rusted one." On zinc and lead it'll be white. Silver is reduced to a black silver sulphide, as described below. The corrosion products that occur on iron and steels appears in a range of reddish, brown to black colours and is known as rust. Some of the blue and green corrosion products you refer to do contain some oxygen but those particular compounds are generally more complex than simple copper tin zinc lead alloys and oxygen. Copper chloride is a turquoise colour CuCl2, no oxidation involved. Copper Nitrate is a good royal blue, if it was only oxygen doing the work the result would be either black or brick red but the nitrogen changes things. Cu(NO3)2 Not all corrosion is in fact a result of oxidisation, as described above. Take silver as an example pertinent to tosogu, it's a silver sulphide Ag2S that forms on the surface and gradually consumes it. I ought to add that the broader topic of corrosion covers much more than only metals and involves many more complex process other than merely oxidisation. The scientific literature on rust lists (at least); 12 varieties of Iron oxides and hydroxides 3 varieties of iron carbonates 9 types of Iron chloride 12 Iron sulphates 4 Iron phosphates and 10 Iron carboxylates and cyanides. These all have their own particular colours and microscopic structures. The colours range from yellows, ochre, green, a wide range of browns through to red, greys and black. I believe it's the interplay of various specific 'rusts' like these, in a patina, that results in the characteristic colour and tone of certain Tanko school's tsuba. What has been especially interesting to me is that traditional tsuba patina recipes and those I've developed from the original sources reflect very well almost all of those varied and complex compounds we generally lump under the generic term 'rust'. Rust is so so much more than Iron oxides. Stable iron patina are invariably complex and multi layered compound structures produced by complex and sophisticated process that were developed over many generations through trial and error. If it just looks like crusty red iron oxide the patina is long gone. Well that'll teach me to throw in my tuppence worth... but as chance would have it I was in fact writing on exactly this topic of corrosion and ferrous patina last evening.1 point
-
He is quite well regarded and from an excellent group of smiths.1 point
-
Hey Mal, I don't have it in hand so I'm not sure how long the nakago is. I can get that next week when I pick it up. The two mekugi do correspond to holes in the nakago. I believe it uses the bottom two for the gunto tsuka. This is why I thought the blade was older because of the one at the top, it looked like the bottom two were either drilled for the conversion or at the very least the middle hole was drilled and it had two originally. When I get it in hand, I'll take some closeup pictures of the holes and we should be able to tell which were drilled.1 point
-
1 point
-
1 point
-
Well I was having bit boring time in a fever, and I won an auction in Japan for a book about swords of the collection of Kurokawa Institute, as they have some amazing items. So an idea came to me that what if I look on some of the most impressive museum collections in Japan and gather that info in a thread. As I have already collected this data actually scooping it together was pretty fast and fun. Here are some absolute top museum collections in Japan, in no particular order. I will mostly list the number of Kokuhō (National Treasure) Jūyō Bunkazai (Important Cultural Property) and Jūyō Bijutsuhin (Important Art Object) swords in the collection. Many of these museums feature amazing items that do not have official designations, and also many have multiple NBTHK designated items. Tōkyō National Museum - https://www.tnm.jp/ Of course it does not need introductions, collection features 19 Kokuhō, 56 JūBu and 6 JūBi. Kyōto National Museum - https://www.kyohaku.go.jp/eng/index.html Similar to above not too much info needed, collection features 3 Kokuhō, 25 JūBu and 2 JūBi. NBTHK - https://www.touken.or.jp/museum/about/collection.html The Japanese Sword Museum houses an amazing collection of items. NBTHK is well known for their appraisal papers. The museum collection includes 3 Kokuhō, 13 JūBu and 13 JūBi, and also several Tokubetsu Jūyō swords. Tokugawa Art Museum - https://www.tokugawa-art-museum.jp/en/about/treasures/sword/ Museum houses over 10,000 artifacts that have been collected by Owari Tokugawa Family. Collection includes 7 Kokuhō, 19 JūBu and 21 JūBi. Sano Art Museum - https://www.sanobi.or.jp/bijutsukan/collection/japanese_sword.html Established by Sano Ryūichi in 1966. I think many know their famous exhibitions and amazing books published on them. Collection includes 2 Kokuhō, 8 JūBu and 36 JūBi also several Tokubetsu Jūyō in collection. Seikado Bunko Art Museum - http://www.seikado.or.jp/en/ Seikado Bunko Library and Art Museum houses Iwasaki family (founder of Mitsubishi group) collection, founder started collecting in 1892. There are 1 Kokuhō, 8 JūBu and 23 JūBi swords in the collection. Eisei Bunko Museum - https://www.eiseibunko.com/index.html Houses the collection of Hosokawa family, public museum was opened in 1973. Collection features 4 Kokuhō, 1 JūBu and 1 JūBi. Kurokawa Insitute of Ancient Cultures - http://www.kurokawa-institute.or.jp/ Research institute established by Kurokawa Family. Collection features 2 Kokuhō, 9 JūBu and 30 JūBi swords. Tōken World - https://www.touken-world.jp/ This newly established museum in Nagoya has acquired stunning collection of items in fairly short time span. Museum was opened in 2020. The collection has 1 Kokuhō, 10 JūBu, 41 JūBi and 58 Tokubetsu Jūyō swords. Japan Sword Museum Technology Research Foundation - http://www.nihontou.or.jp/collection.html This foundation was established in 2015. The collection includes 2 Kokuhō, 20 JūBu, 11 JūBi and 11 Tokubetsu Jūyō swords. Of course there are lots of other amazing museums in Japan that feature excellent swords and related items. And I did not include any shrines and temples in this list (some of them have spectacular items), as I might do a followup list on some of those. The current location of Kokuhō and Jūyō Bunkazai items can be tracked in here: https://kunishitei.bunka.go.jp/bsys/index as I believe their ownership is required to be informed. For Bijutsuhin items tracking down them is lot more trickier but for them I used the historical info that was on the 80's book series that featured every Bijutsuhin sword, as well as more modern info acquired from several Japanese books and magazines. Of course for the bottom 2 new collections it was easy as they list the items they have. They have acquired lot of Bijutsuhin from previously owned privately and other museums. I would also think that after some older owners have passed away some Jūyō Bijutsuhin swords (and Tokujū etc.) would have been donated to Tokyo & Kyoto National Museums, as well as NBTHK and other museums as well. I hope this might be interesting to at least some people. :D1 point
-
Inside the nanban group or Asian export tsuba, this kind of examples I saw listed as Kanton/kagonami tsuba, often with gin zōgan but sometimes with gold.1 point
-
1 point
-
1 point
-
The top row is PHOTO and the second row appears to be a sequence of numbers. I think the photograph is probably coming from an archive, news agency, or collection. Scrap the studio and date comment above. This just goes to show you what happens when I think drink too much!0 points
-
Hi, I was visiting the page Alban indicated out of curiosity and can't resist sharing a different seller's response when asked by one user to show the nakago of what I would consider (as a newbie) a more than dubious Type 94. "Hello, is it possible to have it dismantled? Sincerely" "Hello, once purchased you can take it apart as many times as you like. " Finally, he has ended up uploading a photo.... https://www.naturabuy.fr/shin-gunto-officier-superieur-japonais-lame-damas-tsuba-garnitures-samourai-haute-qualite-item-9320273.html Price: 5 850,00 €0 points
-
All swords need to be tempered. Plus aesthetics. That choji hamon cost me an extra 100 bucks. Suguha would have been cheaper. I could have spent more for a more flamboyant one but I didn’t want to break the bank. The L6 is actually very difficult to work with and any fancier hamon he would have had to make the blade several times to get it right. It would have cost me 1700. He politely asked me not to make him do it as well when we were hammering out the details of the blade. When your buying from a Japanese smith your paying a huge amount for the Japanese laws regarding sword making. They can make three a month I thought it was less. They also have to do an unpaid apprenticeship. They also cannot use any modern methods or metals. Your paying for this extra hard work that is unnecessary with modern metals and equipment. About this video they are destroying nihonto katanas there my friend. Go look up someone doing this to modern 1060 or higher. I am speaking from personal experience. I have destroyed a modern nihonto in a stress test versus this katana. Thats right i said it i destroyed a modern nihonto. Hate me if you must. This L6 toolsteel took everything i threw at it and is perfect and healthy. Europeans did not develop differential hardening anywhere near the same way Japanese smiths did. They used hot tongs and interrupted quenching to achieve the desired flexibility and edge retention. They quench the blade and reheat the spine and quench it again. Japanese swords developed the more advanced hamon to give it a softer spine and harder edge. Thus making their design more flexible than they would have been able to achieve with the process and difficulty involved in separating higher and lower carbon iron. The whole process of making tamahagane is to add carbon to the extremely low carbon iron sand from rivers. As opposed to already high carbon iron mined out if the ground. Once the higher and lower carbon steels are folded the hamons purpose is to make the edge harder and better edge retentive while leaving the spine softer. You just dont need to do all this folding when starting with higher carbon steels. You do still need to temper. All of this technology was driven by necessity. Tamahagane by its very nature has MORE impurities than modern steel. The whole reason for folding the steel is to remove the impurities! You literally do not have to fold modern steel unless you just want to make the blade more traditional looking or prettier. It costs a little more and is entirely unnecessary. I paid extra because i was making something pretty with a hitatsura hamon( the one i just ordered not this one i pictured. ) You cannot fold L6 i am told for any price, they just wont do it. The sulfur in l6 bainite is what makes L6 bainite. Plus a little magnesium i think. Its an alloy and everything in that alloy is supposed to be there. For that matter all steel is an iron/carbon alloy. And to agree with you on one thing YES you’re entirely paying for artistic value. What i am describing is the difference between a ferari and an armored humvee. Yeah the ferari is super nice, very expensive, pretty with all the bells and whistles. Its super exclusive and you need to get in the waiting list for a new one. Who doesnt want a ferari if they had the chance? If you slam the ferari into the armored humvee you’re going to need a new ferari and the armored humvee is gonna keep on trucking. All modern nihinto are works of art to be cherished and appreciated, but if you want something to abuse you definitely dont want to buy that ferari unless you just have that kind of money. Ive already been admonished about this conversation and I honestly appreciate the fact that youre even listening to me and having this discussion. Please dont take me as being rude or derisive. You asked so Im trying to explain my point. Im not buying a crap fake like the ones you see here from time to time made to cheat someone, and thats the only reason i felt the need to say what I did that started this whole conversation between us. It is just a plain cold fact that modern steel and technology can produce a more durable sword than traditional nihonto smithing methods. If you have a problem understanding this i dont know how to better explain what is a simple truth. Your gonna have to buy a high quality modern sword and find out for yourself. @DoTanuki yokai To disagree with this is to disagree with SCIENCE.-1 points
-
Sigh man your arguing with a scientific fact that i have tested myself. There is NOTHING about the traditional process of making a nihonto katana that can match the tolerance achieved with 1065 or higher carbon modern steel. And the carbon is still in the iron they are mining. We’re talking the difference between an lump of iron ore you pull out of the ground and iron ore smelted from RIVER SAND. You’re arguing with the actual historical fact of the reason why Nihonto was developed, because of the poor quality and availability of the iron in Japan. Thats just history.-1 points
-
Seriously this is like arguing with a child who believes in santa claus. The things you are saying are myths. And categorically untrue.-1 points
-
False. The carbon is what is making the steel. The higher the carbon the more flexible and durable the steel. Simple basic science. @DoTanuki yokai And your knowledge is non-existent. Go bend your nihonto katana and cry im done. You linked me a video of nihonto smiths destroying katanas as proof they will stand up to modern steel. I linked you a video of a famous American smith explaining why youre naive and anothed video of high carbon steels being stress tested the same way as your nihonto and passing. At this point youre being willfully ignorant.-1 points
This leaderboard is set to Johannesburg/GMT+02:00